Schrödinger quantum number spin quantum number principal quantum number magnetic quantum number If two electrons in the same atom have the same value of l they are in the same sublevel but not necessarily in the same level. To insert a shape click a shape in the shapes ____.

Solved Which Of The Following Quantum Numbers Describes The Chegg Com
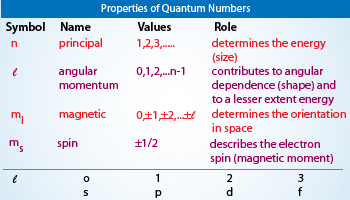
A brief description of each of these numbers in the set of four quantum numbers that describe the unique quantum state of an electron in atomic physics can be found below.

. This is a very rough explanation. First of all you should know that azimuthal quantum number in earlier times was denoted by k which had value equal to l1. 3 only O d.
Principle quantum number describes. Thus s orbital corresponds to spherical shape with the atomic nucleus at its centre. B the angular momentum quantum number l describes the size and energy associated with an orbital.
For every value of n there is one s orbital ie. In chemistry and spectroscopy ℓ 0 is called an s orbital ℓ 1 a p orbital ℓ 2 a d orbital and ℓ 3 an f orbital. What is the maximum number of electrons that can occupy a box in an orbital filling diagram.
An _____ defines a two-dimensional shape. Size of the orbital. Azimuthal quantum number ie.
Shape of orbital b. Azimuthal quantum number describes the shape of the orbital and represented by l and spin quantum number describes the spin of the electron and magnetic quantum number describes the orientation of the electron cloud in the orbital. The correct option is ii.
No two electrons will have the four quantum numbers as the same. It is denoted by the symbol l and its value is equal to the total. Angular momentum quantum number l describes the shape of the orbitals.
Share Improve this answer. Which of the following quantum numbers describes the shape of an orbital. An orbital is the path that an electron follows during This problem has been solved.
Orientation in the orbital electron cloud. You need to learn something like quantum chemistry or quantum mechanics in order to get a better understanding on that. Magnetic quantum number which is denoted by ml m l is used to specify its orbital.
It also gives the shape of orbitals. What is the shape of the s orbital. 1 only O b.
Which of the following quantum numbers describes the shape of an orbital. The azimuthal quantum number is just the quantum number used to describe angular part of the wave function so it determines the shape of the orbital. Orientation of in the orbitalelectron cloud.
Principal Quantum number describes- a. Which quantum number describes the shape of the orbital. S orbitals are present in all principal energy levels.
The principal quantum number indicates what property of an electron. C the magnetic quantum number ml describes the orientation of the orbital. The angular momentum quantum number describes the the size and energy associated with an orbital.
The ratio of nk denoted the shape of the orbital If nk 1 it will be circular. Each element has an electron configuration that can be expressed in quantum numbers Each orbital in an atom consists of 4 quantum numbers each describing the state of the orbital n the principal quantum number. A the principle quantum number n describes the shape of an orbital.
The principal quantum number n describes the shape of an orbital b. Azimuthal quantum number which is denoted l l is used to specify its subshell. L determines the shape and size of the orbital.
Read More Trending doubts. In a px orbital the subscript x denotes the _____ of the electron. The shape of an orbital is defined by the angular momentum quantum number which is represented as letter _____ asked Sep 12 2016 in Chemistry by TTCOXO general-chemistry.
If nk 21 it will be elliptical. In the same orbital. What value of l is represented by an g orbital.
The magnetic quantum number m describes the orientation of the orbital. We can think about it this way. Size of the orbital.
The azimuthal or orbital angular momentum quantum number describes the shape of a given orbital. The second quantum number known as the angular or orbital quantum number describes the subshell and gives the magnitude of the orbital angular momentum through the relation. Size of the orbital c.
The magnetic quantum number m is related to the orientation in space of an orbital within a given subshell. Spin of an electron. Two aqueous solutions are both at room temperature and are.
Angular momentum quantum number. For s orbital Azimuthal quantum number 0 and the magnetic quantum number m 0 hence s orbitals have unique orientation in space. A 1 B 2 C 0 D 3 E 4 26.
No more than two electrons in an atom can have the same principal quantum number n. 1 2 and 3 e. 2 only O c.
The size of the orbitals. The principal quantum number n describes the shape of an atomic orbital. A magnetic quantum number B principal quantum number C angular momentum quantum number D spin quantum number E Schrödinger quantum number 25.
10th - 11th grade. The angular momentum quantum number is a quantum number that describes the shape of an orbital and tells us which subshells are present in the principal shell. Spin of electron d.

0 Comments